A temporally consistent atlas of neonatal brain development
Excited to present my work on temporally consistent modelling of the neonatal brain morphology from structural brain magnetic resonance (MR) images at the Centre for the Developing Brain at King’s College London, St’ Thomas Hospital.
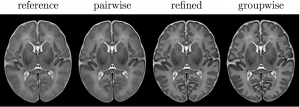
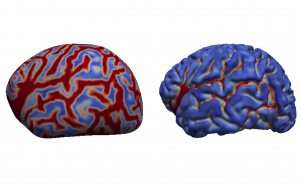
After providing some background on brain atlas construction, the talk focuses on our novel group-wise approach for the construction of an unbiased spatio-temporal brain with improved temporal consistency, lower computational cost, and considerably higher cortical detail than previous neonatal atlas construction techniques. It is a summary of our work detailed in Schuh et al., “Unbiased construction of a temporally consistent morphological atlas of neonatal brain development”, preprint available on bioRxiv (doi:10.1101/251512).